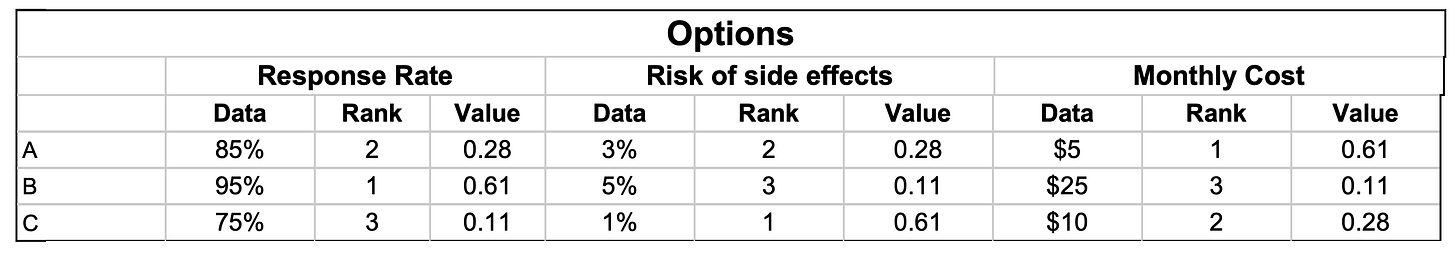
A frequently neglected component of shared decision making
At its core, shared decision making is a process in which decisions are made in a collaborative way, where trustworthy information is provided in accessible formats about a set of options, typically in situations where the concerns, personal circumstances, and contexts of patients and their families play a major role in decisions. [1]
Recently I was asked to help a friend understand the results of a new biomarker test. He had been told his result was abnormal but, beyond that, was not able to find any additional information that would help him understand how to use the test result to help decide if additional testing is warranted.
The test manufacturer’s website states that, using the recommended cutoff value, the test has a 91% negative predictive value and a sensitivity of 92%, but has no information about the test specificity used to derive the negative predictive value. Fortunately there was a link on the website to the paper used to derive both the reported 92% test sensitivity and the test specificity used to calculate the negative predictive value, which is 30.1%. With this information I was able to quickly determine that his chance of having a significant problem warranting additional workup is somewhere between 10% and 25% based on what I thought his pre-test probability might be.
For patients to adequately participate in managing a problem, they need to know what the possible diagnoses are and how certain, or uncertain, they are. The role of diagnostic tests is to provide information about disease likelihoods in an understandable manner that can directly help people make good management decisions.
Currently, the best way to characterize decision related uncertainties is through the use of probabilities. Calculating or estimating diagnostic probabilities that result from a diagnostic test requires a basic understanding of Bayes Theorem and its proper application. Bayes theorem was derived in 1763 by Reverend Thomas Bayes. [2] It was introduced into Medicine in a 1959 paper by Ledley and Lusted [3] and popularized in a series published in the Annals of Internal Medicine in the early 1980’s. [4] Although frequently taught to medical students, my impression is that it is just as frequently forgotten and rarely used in clinical practice. From what I have seen, it is also not applied in diagnostic test research studies in a way that will inform clinical decisions. Most publications seem to focus on the test and fail to provide information useful for clinicians and patients wishing to understand how to use test results to make manage clinical problems.
Using Bayes Theorem to determine the likelihood of disease following a test result requires three pieces of information: test sensitivity (the proportion of people known to have disease who have a positive test), test specificity (the proportion of people without disease who have a negative test), and the probability of disease before the test results are known – the pre-test probability. Test sensitivities and specificities are determined by comparing test results with an unrelated test or other way of establishing the presence or absence of disease. The pre-test probability is usually considered a subjective probability that measures the strength of belief that the disease is present. For some conditions diagnostic calculators are available that estimate disease probability based on collections of clinical variables. If available, they are excellent ways to estimate pretest probabilities. Once the three key quantities are known, the probability of disease following a positive or negative test result can be easily computed. There are a host of Bayesian calculators freely available online. I particularly liked the one at Betterexplained.com.
Musings
Failure to use Bayes theorem to provide clinicians and patients with probabilistic information about diagnostic test results is both a shame and completely unnecessary.
References
1. Elwyn G, Durand MA, Song J, Aarts J, Barr PJ, Berger Z, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891
2. Bayes’ theorem. https://en.wikipedia.org/wiki/Bayes%27_theorem
3. Ledley RS, Lusted LB. Reasoning Foundations of Medical Diagnosis. Science 1959;130.
4. Griner, P. F., Mayewski, R. J., Mushlin, A. I., & Greenland, P. (1981). Selection and interpretation of diagnostic tests and procedures. Annals of Internal Medicine, 94(4 II).